

Milk is a promotor of growth and anabolism via activating insulin-like growth factor-1 (IGF-1)/phosphatidylinositol-3 kinase (PI3K)/AKT/mechanistic target of rapamycin complex 1 (mTORC1) signaling.
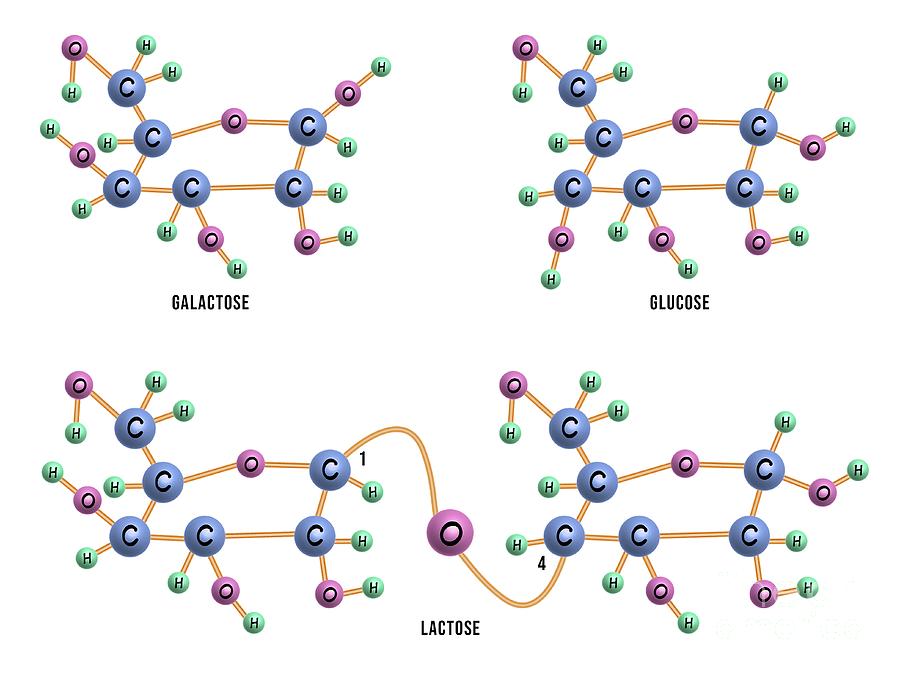
#Glucose and lactose windows
The potential impact of cow milk consumption on the pathogenesis of PCa may already begin during fetal and pubertal prostate growth, critical windows with increased vulnerability. Epidemiological studies identified commercial cow milk consumption as a potential risk factor of PCa. Articles in PubMed until November 2021 reporting on milk intake and PCa were reviewed. This review analyzes the potential impact of milk-induced signal transduction on the pathogenesis of prostate cancer (PCa). Via the vagal nerve and/or systemic exosomes, toxic α-syn may spread to dopaminergic neurons and pancreatic β-cells linking the pathogenesis of PD and T2D. MiRNA-148a- and galactose-induced mitochondrial oxidative stress activate c-Abl-mediated aggregation of α-syn which is exported by exosome release. The potential uptake of MEX miRNA-148a and miRNA-21 by enteroendocrine cells in the gut, dopaminergic neurons in substantia nigra and pancreatic β-cells may enhance miRNA-148a/DNMT1-dependent overexpression of α-syn and impair miRNA-148a/PPARGC1A- and miRNA-21/LAMP2A-dependent autophagy driving both diseases. MEX are taken up by intestinal epithelial cells and accumulate in the brain after oral administration to mice. This review provides translational evidence that milk exosomes (MEX) disturb α-syn homeostasis. Exosomes are critical transmitters of α-syn from cell to cell especially under conditions of compromised autophagy. Prion-like vagal nerve-mediated propagation of exosomal α-syn from the gut to the brain and pancreatic islets apparently link both pathologies. In T2D, α-syn promotes co-aggregation with islet amyloid polypeptide in pancreatic β-cells. PD is an α-synucleinopathy associated with mitochondrial dysfunction, oxidative stress, deficient lysosomal clearance of α-synuclein (α-syn) and aggregation of misfolded α-syn.

These two taste attributes dominated in liking the lactose-free kefir by elderly subjects.Įpidemiological studies associate milk consumption with an increased risk of Parkinson’s disease (PD) and type 2 diabetes mellitus (T2D). The lower acidity of LFK than that of TK, assayed both instrumentally and by sensory assessment, was highly appreciated by 73% of elderly subjects as “just about right” in JAR scale. The intense sweet taste of LFK was due to higher amounts of glucose and galactose than in TK, and was perceived as “just about right” by 63% of elderly subjects in the just-about-right (JAR) scale. LFK was sensed as sweeter and more milky than the traditional one. A substantial share of acetic acid in LFK was not associated with high intensity of sour aroma, probably being masked by the creamy aroma, perceived as dominating. The LFK contained two times more ketones, especially 3-hydroxy-2-butanone and 2,3-butanedione, that probably contributed to the high intensity of creamy aroma. The perception of main sensory attributes and hedonic acceptability of LFK by elderly were also studied. The study aimed at determining the volatile compound profile and the corresponding sensory attributes of lactose-free kefir (LFK) as compared with the traditional one (TK). Lactose-free products are crucial in the diet of lactose-intolerant elderly consumers, one of them being kefir due to its unique chemical composition and diversity of valuable microflora.


 0 kommentar(er)
0 kommentar(er)
